INFECTIOUS DISEASES
Infectious diseases. Infectious diseases are caused by pathogenic microorganisms, such as bacteria, viruses, parasites or fungi; the diseases can be spread, directly or indirectly, from one person to another. Zoonotic diseases are infectious diseases of animals that can cause disease when transmitted to humans.
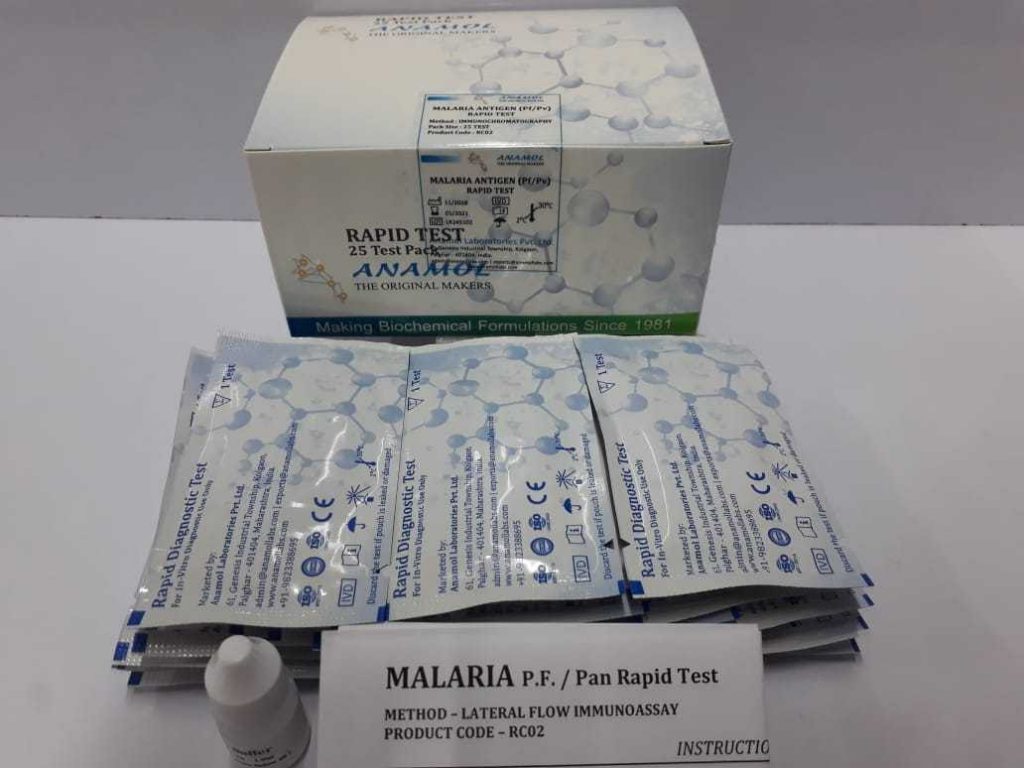
MALARIA Pf/Pv ANTIGEN CARD TEST
Intended Use:
Test for qualitative detection of Malaria P. falciparum and P. vivax in human whole blood samples.
Introduction:
Malaria is a serious parasitic disease characterized by fever, chills and anemia and is caused by a parasite that is transmitted from one human to another by the bite of infected Anopheles mosquitoes. There are four kinds of malaria that can infect humans: Plasmodium falciparum, P. vivax, P. Ovale, and P. Malaria. In humans, the parasites (called sporozoites) migrate to the liver where they mature and release another form, the merozoites.
Principle:
The Malaria Antigen Test contains a membrane strip, which is pre-coated with two monoclonal antibodies as two separate lines across a test strip. One monoclonal antibody (test line 1) is specific to the P. falciparum histidine rich protein-2 (pf HRP II) and another monoclonal antibody (test line 2) is pan specific to lactate Dehydrogenase (Pan LDH) of plasmodium species (P.Falciparum, Vivax, Malaria, Ovale). Conjugate pad is dispensed with monoclonal antibodies conjugated to the colloidal gold, which are specific to P. Falciparum histidine rich protein-2(pf HRP-II) and pan specific to the Lactate Dehydrogenase (Pan LDH) of other Plasmodium species.
Pack Size:
25 Test.
50 Test.
Kit components:
Test Device.
Assay Buffer.
Instructions for Use.
Storage Temperature:
Room Temperature (2-30 0C).
Shelf life:
24 months
Sample Type:
Whole Blood only collected in EDTA, Heparin or Cit-rate anticoagulants.
Test Procedure:
Qualitative only.
Sample Volume:
5 µl.
Procedure Time:
20 to 25 minutes.
Sensitivity:
100% for Pf as well as Pv.
Specificity:
99.8%.
Availability:
In Stock.
Dispatch time in weeks:
2 to 4 weeks.
Kit dimensions:
25 test – 75x150x110 mm.
50 test – 75x275x135 mm.
Approx weight of kit:
25 test – 150 gms.
50 test – 300 gms.
No of kits in shipper:
25 Test – 32 kits.
50 Test – 24 kits.
Dimension of shipper:
410x550x378 mm.
Approx weight of shipper:
25 test – 6 kgs.
50 test – 8.5 kgs
Samples availability:
Yes.
Intended use:
Test for determination of antibodies against Salmonella antigens by Slide & Tube Test method.
Introduction:
Salmonella typhi & Salmonella paratyphi are the causative agents of “Enteric Fever”. The antigens of typhoid and paratyphoid consist of 2 distinct fractions – the stable somatic ‘O’ antigen and the labile flagellar ‘H’ antigen. The paratyphoid antigens are further classified into ‘A’ and ‘B’ species. In typhoid and paratyphoid, the ‘H’ antigen is type specific whereas the ‘O’ antigen is group specific.
Method Principle:
Widal antigens are the standardized smooth suspension of killed bacterial antigens of qualitative and semi-quantitative detection of S. typhi and S. paratyphi antibodies. An undiluted serum is used in slide test. It is simple, rapid and convenient screening test. The slide test is standardized in such a way that they can be used for either slide or tube technique. In doubtful cases, it is recommended to perform the tube technique for obtaining conclusive results. A marked rise in the titer to one serotype (above 1:80) suggests infection. Diagnostically, a rising antibody titer of at least four-fold (two tube difference) is considered more significant than a single test. It is observed that individuals immunize with TAB vaccine may show a moderately high titer for all antigens.
Pack Size:
2×5 ML.
2+2×5 ML.
Kit components: 2×5 ml
S. typhi ‘O’ Antigen – 1 bottle of 5 ml.
S. typhi ‘H’ Antigen – 1 bottle of 5 ml.
Positive Control – 1 bottle.
Glass Slide – 1 Nos.
Instructions for Use.
Kit components: 2+2×5 ml
S. typhi ‘O’ Antigen – 2 bottles of 5 ml each.
S. typhi ‘H’ Antigen – 2 bottles of 5 ml each.
Positive Control – 1 bottle.
Glass Slide – 1 Nos.
Instructions for Use.
Storage Temperature:
2- 8 0C.
Shelf life:
18 months.
Sample Type:
Serum only.
Availability:
In Stock.
Dispatch time in weeks:
2 to 4 weeks.
Procedure:
Qualitative only (Slide Test), Semi-quantitative method (Slide Test) as well as Quantitative method (Tube Test).
Procedure Time:
1 min for qualitative and semi-quantitative method.
Sensitivity:
Up to 1:320 dilutions in Semi-quantitative and Quantitative test method.
Kit dimensions:
2×5 ml – 85x105x45 mm.
2+2×5 ml – 85x105x45 mm.
Approximate weight of kit:
2×5 ml – 77 gms.
2+2×5 ml – 107 gms.
No of kits in shipper:
2×5 ml – 92 kits.
2+2×5 ml – 92 kits.
Dimension of shipper:
410x550x378 mm
Approximate weight of shipper:
2×5 ml – 10 kg.
2+2×5 ml – 13 kg.
Samples availability:
Yes.

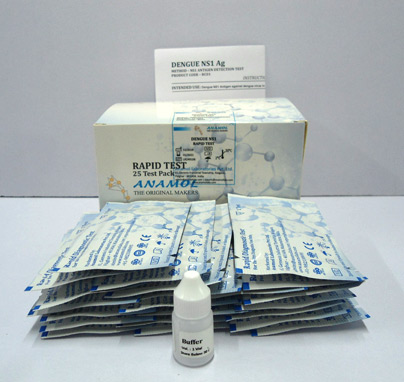
Dengue Combo Card Test
WIDAL 2+2x5 ML / 2x5 ML
Intended Use:
Test for detection of both NS1 antigen and IgG/IgM antibody against Dengue virus in serum and plasma.
Introduction:
The NS1 antigen is expected to be detected 1 day after the onset of fever and persist upto 9 days in both primary and secondary dengue infection. But the detection of NS1 is inhibited, if anti- NS1 is produced. Primary dengue is characterized by the presence of detectable IgM 3-4 days after the onset infection. Secondary dengue is characterized by the elevation of IgG 1-2 days after the onset infection and in majority of cases this is generally accompanied by an elevation of IgM.
Method Principle:
The Dengue NS1 Antigen Test Device (Serum/ Plasma/Whole Blood) is a qualitative test for the detection of NS1 antigen to dengue virus in human serum or plasma. Only serum and plasma samples may be used with this test.
For NS1 Antigen: First a specimen is dispensed buffer; the Gold antibody conjugate will bind to Dengue antigen in the specimen sample which in turn will bind with Anti-Dengue NS1 coated on the membrane. As the reagent moves across the membrane, the Dengue NS1 antibody on the membrane will bind the antibody-antigen complex causing pale or dark pink line to form at the test line region of the test membrane. The intensity of the lines will vary depending upon the amount of antigen present in the sample. The appearance of pink line in the test region should be considered as positive result.
For IgG/IgM Antibodies: First a specimen is dispensed with sample buffer, the Gold antigen conjugate will bind to anti- Dengue IgG and IgM antibodies in the specimen sample which in turn will bind with Anti-Human IgG and Anti-Human IgM coated on the membrane as two separate lines in the test region as the reagent move across the membrane. The anti-Human antibodies on the membrane will bind the IgG or IgM antigen complex at the relevant IgG and or IgM test lines causing pale or dark pink lines to form at the IgG or IgM region of the test membrane. The intensity of the lines will vary depending upon the amount of antibody present in the sample.
Pack Size:
25 Test.
50 Test.
Kit components:
Test Device.
Assay Buffer – Separate buffer for Antigen and Antibody.
Sample Dropper.
Instructions for Use.
Sample Type:
Serum or Plasma.
Storage Temperature:
Room Temperature (2-30 0C).
Shelf life:
24 months
Test Procedure:
Qualitative only.
Sample Volume:
10 µl for NS1 Antigen.
5 µl for IgG/IgM Antibody.
Buffer Volume:
30 µl (1 drop) for NS1 Antigen.
60 µl (2 drops) for IgG/IgM Antibody.
Procedure Time:
15 to 30 Minutes.
Availability:
In Stock.
Dispatch time in weeks:
2 to 4 weeks.
Kit dimensions:
25 test – 75x150x110 mm
50 test – 75x275x135 mm
Approx weight of kit:
25 test – 150 gms
50 test – 300 gms
No of kits in shipper:
25 Test – 32 kits
50 Test – 24 kits
Dimension of shipper:
410x550x378 mm
Approx weight of shipper:
25 test – 6 kgs
50 test – 8.5 kgs
Samples availability:
Yes
Production Capacity:
50 mn tests per annum.
Others Include:
- Chagas Rec
- Chylamadia Trachomatis
- CMV
- Denge
- EBV
- H. pylori
- HBsAg
- anti-HBc
- anti-HBs
- anti-HCv
- anti-HIV-1/2
- HIV-Ag/Ab
- HSV
- Measles virus
- Mumps virus
- Rubella virus
- Syphilis screen
- Toxo
- V
